AUGER Electron Spectroscopy Should not be confused with Atomic emission Spectroscopy. AES is a popular method in material analysis.
The Auger effect is an electronic process at the heart of AES resulting from the inter- and intrastate transitions of electrons in an excited atom. When an atom is probed by an external mechanism, such as a photon or a beam of electrons with energies in the range of 2 KeV to 50 KeV, a core state electron can be removed leaving behind a hole. As this is an unstable state, the core hole can be filled by an outer shell electron, whereby the electron moving to the lower energy level loses an amount of energy equal to the difference in orbital energies. The transition energy can be coupled to a second outer shell electron which will be emitted from the atom if the transferred energy is greater than the orbital binding energy.An emitted electron will have a kinetic energy of:
The Auger effect is an electronic process at the heart of AES resulting from the inter- and intrastate transitions of electrons in an excited atom. When an atom is probed by an external mechanism, such as a photon or a beam of electrons with energies in the range of 2 KeV to 50 KeV, a core state electron can be removed leaving behind a hole. As this is an unstable state, the core hole can be filled by an outer shell electron, whereby the electron moving to the lower energy level loses an amount of energy equal to the difference in orbital energies. The transition energy can be coupled to a second outer shell electron which will be emitted from the atom if the transferred energy is greater than the orbital binding energy.An emitted electron will have a kinetic energy of:
where  ,
, 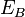 ,
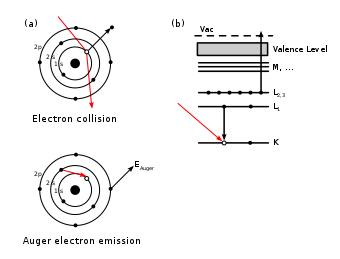
,  are respectively the core level, first outer shell, and second outer shell electron energies, measured from the vacuum level. The apostrophe (tic) denotes a slight modification to the binding energy of the outer shell electrons due to the ionized nature of the atom; often however, this energy modification is ignored in order to ease calculations.Since orbital energies are unique to an atom of a specific element, analysis of the ejected electrons can yield information about the chemical composition of a surface. Figure 1 illustrates two schematic views of the Auger process.
are respectively the core level, first outer shell, and second outer shell electron energies, measured from the vacuum level. The apostrophe (tic) denotes a slight modification to the binding energy of the outer shell electrons due to the ionized nature of the atom; often however, this energy modification is ignored in order to ease calculations.Since orbital energies are unique to an atom of a specific element, analysis of the ejected electrons can yield information about the chemical composition of a surface. Figure 1 illustrates two schematic views of the Auger process.
 ,
, 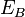 ,
,  are respectively the core level, first outer shell, and second outer shell electron energies, measured from the vacuum level. The apostrophe (tic) denotes a slight modification to the binding energy of the outer shell electrons due to the ionized nature of the atom; often however, this energy modification is ignored in order to ease calculations.Since orbital energies are unique to an atom of a specific element, analysis of the ejected electrons can yield information about the chemical composition of a surface. Figure 1 illustrates two schematic views of the Auger process.
are respectively the core level, first outer shell, and second outer shell electron energies, measured from the vacuum level. The apostrophe (tic) denotes a slight modification to the binding energy of the outer shell electrons due to the ionized nature of the atom; often however, this energy modification is ignored in order to ease calculations.Since orbital energies are unique to an atom of a specific element, analysis of the ejected electrons can yield information about the chemical composition of a surface. Figure 1 illustrates two schematic views of the Auger process.
Figure 1. Two views of the Auger process. (a) illustrates sequentially the steps involved in Auger deexcitation. An incident electron creates a core hole in the 1s level. An electron from the 2s level fills in the 1s hole and the transition energy is imparted to a 2p electron which is emitted. The final atomic state thus has two holes, one in the 2s orbital and the other in the 2p orbital. (b) illustrates the same process using spectroscopic notation,  .
.
 .
.