Luminance is a photometric measure of the luminous intensity per unit area of light travelling in a given direction. It describes the amount of light that passes through, is emitted from, or is reflected from a particular area, and falls within a given solid angle.
Illuminance is the total luminous flux incident on a surface, per unit area.[1] It is a measure of how much the incident light illuminates the surface, wavelength-weighted by the luminosity function to correlate with human brightness perception.[2] Similarly, luminous emittance is the luminous flux per unit area emitted from a surface. Luminous emittance is also known as luminous exitance.[3][4]
Irradiance: The amount of energy incident on a given area of a surface in a given amount of time (W/m2).
Irradiance is the radiometry term for the power per unit area of electromagnetic radiation incident on a surface. The SI unit for irradiance is watts per square meter [W/m2], or milliwatts per square millimeter [mW/mm2]. (Irradiance is sometimes called intensity, but this usage leads to confusion with another standard, but infrequently used, radiometry unit —Radiant Intensity — which is measured in watts per steradian.)
If a point radiation source emits radiation uniformly in all directions and there is no absorption, then the irradiance drops off in proportion to the distance squared from the source, since the total power is constant and it is spread over an area that increases with the distance squared from the radiation source. To compare the irradiance of different sources, one must take into account the distance from the source. A 50 cm distance is often used for such measurements.
Irradiance is a useful measure for applications where power must be delivered to large areas. For example, illuminating a classroom or a football field is primarily a question of delivering a certain number of watts per square meter. This can be achieved by using a single high power source. However, since irradiance does not depend on solid angle, multiple sources can be combined, illuminating the walls or the field from different angles.
The irradiance of a source is not the most useful measure when designing an efficient optical coupling system that collects radiation from a source, and then delivers the radiation into an optical instrument. Such optical instruments will have a limited entrance aperture and a limited acceptance solid angle. In such cases it is the radiance of the source (its ‘brightness’) that is most useful.
Radiance: The amount of energy scattered in a particular direction (W/m2/sr).
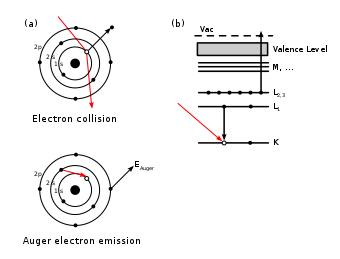
The SI unit of radiance is watts per square meter per steradian [W/m2-sr]. Since many radiation sources used in laboratories have emitting area in the square millimeters range, the unit of milliwatts per square millimeter per steradian [mW/mm2-sr] is often used for radiance. As shown in Figure 1, the radiance (R) of the source emitting area (A) equals the radiation power (P), which is emitted from A and propagates in solid angle Ω, divided by the area A and the solid angle Ω: R = P / (A x Ω).
Tech-understanding-radiance-image
Figure 1. (left) Radiance (R) of source is the Power (P) emitted from the source emitting Area (A) and propagated in the Solid Angle (Ω).
The steradian [sr] is the SI unit for measuring solid angles, defined by the solid angle (Ω) that projects on the surface of a sphere with a radius of r, having an area (A) equal to r2 (Ω = A/r2 = r2/r2 = 1 [sr]). It describes angular spans in three-dimensional space, analogous to the way in which the radian [rad] describes angles in a two-dimensional plane. The total solid angle for a point in space is 4π steradians.
Steradian
Figure 2. (left) Steradian [sr] is a unit for measuring solid angles (Ω) defined by the solid angle that projects on the surface of a sphere, with a radius of r, having an area of A = r2 (Ω = A/r2 = r2/r2 = 1 [sr]).
The radiance of a source is increased by increasing its emitted power, by making the emitting area of the source smaller or by emitting the radiation into a smaller solid angle. Strictly speaking, radiance is defined at every point on the emitting surface, as a function of position, and as a function of the angle of observation. Often, as in the example above we use radiance of a source to mean the radiance averaged over a finite sized aperture and over some solid angle of interest.
Radiance is a conserved quantity in an optical system so that radiance measured as watts per unit area per unit solid angle incident on a detector will not exceed the radiance at the emitter. In practice, for any bundle of rays mapping an emitter to a detector, the radiance seen at the detector will be diminished by the light which is absorbed along the way or scattered out of the solid angle of the bundle of rays reaching the detector.
Let us consider an example. Suppose one observes with the eye a 35W Xenon (Xe) short-arc lamp, and then a 60W straight tube fluorescent lamp, both at a similar distance of a few meters. (As background information, the 35W arc lamp emits significantly less visible power than the 60W fluorescent tube.) Which light source is perceived to be brighter, or in radiometric terms, has higher radiance? The Xe short-arc lamp is perceived to be much brighter, although the 35W arc lamp emits less power than the 60W fluorescent lamp. This is as a result of the much smaller emitting area (A) of the short-arc lamp compared to the very large emitting area of the fluorescent lamp, while the eye is receiving the radiation at more or less the same solid angle (Ω) when the distance between the eye and the source is the same. The eye’s lens forms a bright image of the Xe arc on a very small area of the retina and the eye does not feel comfortable. The larger area fluorescent lamp will form an image over a much larger area on the retina, which the eye can tolerate more comfortably. The arc-lamp has a much higher radiance than the fluorescent lamp, even though it emits less power.
By way of a further example, imagine using the Xe and fluorescent lamps to illuminate a small area such as the end of a 200 μm diameter optical fiber. As a result of the higher source radiance the radiation from the 35W Xe arc-lamp can be much more efficiently collected and focused into the fiber. In contrast, the low radiance 60W fluorescent lamp will be ineffective in coupling its radiation energy into the fiber, no matter what type of focusing optic is used.
Energetiq’s Laser-Driven Light Sources have ultrahigh radiance from their small emitting area (~ 100 μm diameter). Radiation from such a high radiance and small emitting area source can be even more efficiently coupled into the 200 μm diameter optical fiber described above. This is also true for other optical systems with small apertures and a limited accepting solid angle - optical systems with small ‘étendue’ - such as the narrow slits of a monochromator. (For further discussion of étendue, see Application Note #002-2-14-2011, Etendue and Optical Throughput Calculations.)
Radiant flux is radiant energy per unit time, also called radiant power [W, mW or μW]. Radiant flux is often used to describe the radiation power output of a radiation source, or the radiation power received by an optical instrument. Examples of radiant flux are: the radiation power passing through a pinhole; the radiation power emerging from the optical fiber of a fiber-coupled laser; the radiation power received by a power detector.
Non-standard terms such as brightness, radiant power, flux, and intensity
Radiance, Irradiance and Radiant Flux,
1. NonStandard
2. Photometry terms
3. Radiometry terms
| Quantity | Unit | Dimension | Notes | |||||
|---|---|---|---|---|---|---|---|---|
| Name | Symbol[nb 1] | Name | Symbol | Symbol[nb 2] | ||||
| Luminous energy | Qv[nb 3] | lumen second | lm⋅s | T J | The lumen second is sometimes called the talbot. | |||
| Luminous flux, luminous power | Φv[nb 3] | lumen (= candela steradian) | lm (= cd⋅sr) | J | Luminous energy per unit time | |||
| Luminous intensity | Iv | candela (= lumen per steradian) | cd (= lm/sr) | J | Luminous flux per unit solid angle | |||
| Luminance | Lv | candela per square metre | cd/m2 (= lm/(sr⋅m2)) | L−2J | Luminous flux per unit solid angle per unit projected source area. The candela per square metre is sometimes called the nit. | |||
| Illuminance | Ev | lux (= lumen per square metre) | lx (= lm/m2) | L−2J | Luminous flux incident on a surface | |||
| Luminous exitance, luminous emittance | Mv | lumen per square metre | lm/m2 | L−2J | Luminous flux emitted from a surface | |||
| Luminous exposure | Hv | lux second | lx⋅s | L−2T J | Time-integrated illuminance | |||
| Luminous energy density | ωv | lumen second per cubic metre | lm⋅s/m3 | L−3T J | ||||
| Luminous efficacy (of radiation) | K | lumen per watt | lm/W | M−1L−2T3J | Ratio of luminous flux to radiant flux | |||
| Luminous efficacy (of a source) | η[nb 3] | lumen per watt | lm/W | M−1L−2T3J | Ratio of luminous flux to power consumption | |||
| Luminous efficiency, luminous coefficient | V | 1 | Luminous efficacy normalized by the maximum possible efficacy | |||||
| See also: SI · Photometry · Radiometry | ||||||||
References:
https://www.energetiq.com/technote-understanding-radiance-brightness-irradiance-radiant-flux



 ,
, 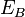 ,
,  are respectively the core level, first outer shell, and second outer shell electron energies, measured from the vacuum level. The apostrophe (tic) denotes a slight modification to the binding energy of the outer shell electrons due to the ionized nature of the atom; often however, this energy modification is ignored in order to ease calculations.Since orbital energies are unique to an atom of a specific element, analysis of the ejected electrons can yield information about the chemical composition of a surface. Figure 1 illustrates two schematic views of the Auger process.
are respectively the core level, first outer shell, and second outer shell electron energies, measured from the vacuum level. The apostrophe (tic) denotes a slight modification to the binding energy of the outer shell electrons due to the ionized nature of the atom; often however, this energy modification is ignored in order to ease calculations.Since orbital energies are unique to an atom of a specific element, analysis of the ejected electrons can yield information about the chemical composition of a surface. Figure 1 illustrates two schematic views of the Auger process.